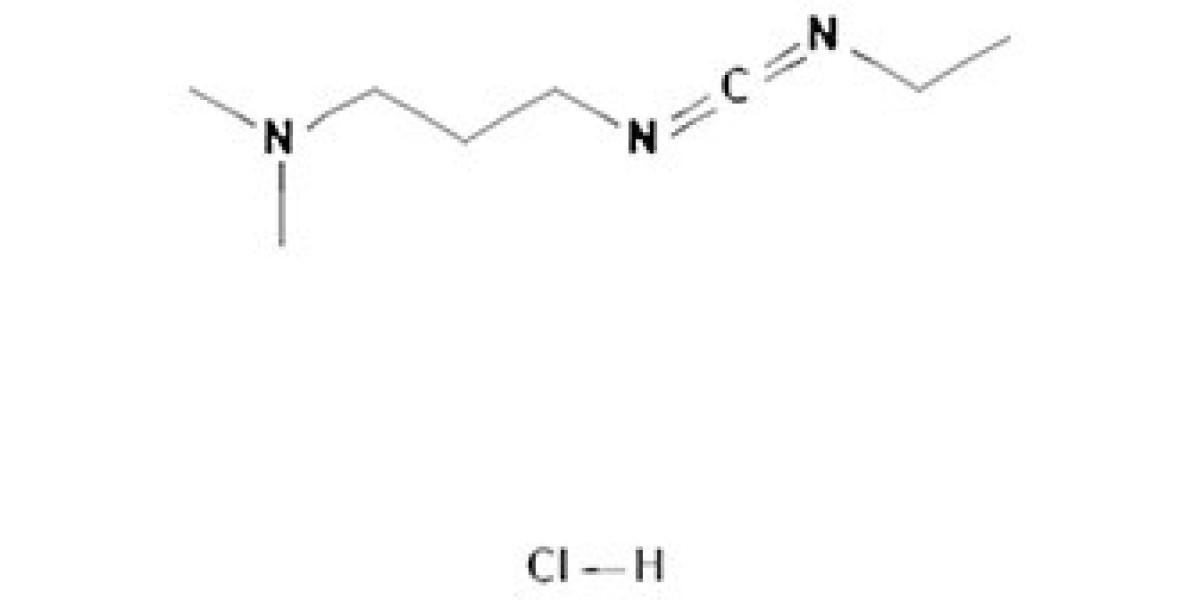
In the field of chemical synthesis, the demand for dependable intermediates continues to grow. Among the most reliable options available today, EDC HCL stands out for its stable performance and its essential role in supporting controlled, high-precision reactions across multiple industries.
The Role of EDC HCL in Chemical Development
EDC HCL (1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride) is valued for its ability to activate carboxyl groups effectively, making it a preferred choice in peptide synthesis and organic chemistry. Its clean reaction profile and consistent behavior make it suitable for both research and scaled production.
Key Strengths of EDC HCL
Ensures dependable reaction effectiveness
Supports clean activation with minimal by-products
Suitable for both laboratory and industrial conditions
Stable, easy to handle, and reliable in varied environments
Applications Across Key Sectors
1. Pharmaceutical Manufacturing
EDC HCL plays a significant role in forming intermediates that support API development. Its precision helps maintain safety and consistency in sensitive chemical pathways.
2. Research Laboratories
Researchers depend on EDC HCL for controlled experiments, reproducible outcomes, and reliable synthesis steps.
3. Fine and Specialty Chemicals
The compound contributes to the development of advanced materials, dyes, and specialized compounds where consistent performance is required.
Why Industries Rely on Lifechem Pharma
Lifechem Pharma focuses on supplying high-quality chemical intermediates that meet strict quality standards. Each batch undergoes careful evaluation to ensure uniformity, safety, and efficiency, supporting industries that require accuracy at every stage of production.
The Importance of Complementary Intermediates
Along with EDC HCL, industries often use supporting compounds such as methyl carbazate, which enhances reaction flexibility and supports the development of complex chemical structures.
Conclusion
EDC HCL remains an essential component in advancing chemical synthesis across pharmaceuticals, research organizations, and specialty chemical manufacturing. Its stability, efficiency, and versatility make it a dependable choice for controlled and precise reactions. When paired with complementary materials such as methyl carbazate, industries gain a strengthened foundation that supports innovation and ensures consistent performance at every stage of development.
Frequently Asked Questions
1. What is EDC HCL mainly used for?
It is primarily used as a coupling agent in peptide and organic synthesis.
2. Why is EDC HCL preferred in pharmaceutical applications?
Its clean reaction profile and reliable activation support safe and precise pharmaceutical development.
3. Is EDC HCL suitable for laboratory research?
Yes. Its consistency makes it ideal for controlled experiments and academic or industrial research.
4. What is the role of methyl carbazate?
Methyl carbazate acts as an important intermediate in forming hydrazine-based compounds used in various chemical processes.
5. Why choose Lifechem Pharma for these intermediates?
Lifechem Pharma ensures strict quality control, stable supply, and dependable performance, making it a trusted source for essential chemical materials.